Polyethylene is one kind of polymer that very popular plastic in the world, this kind of polymer usually makes shampoo bottles, soap bottles, tail lamps, inner panels, electrical and mechanical gear, children toys, grocery bags and little electrical parts components like in printer, vacuum cleaner and others , poly mean “ many” and mer is part, so we can easy define polymer as many parts and the molecule of polymer has usually repeatedly in a chain-like manner. Those parts is called monomers, same structural monomer connected by covalent chemical bound.

Furthermore, Polyethylene is many ethylene that bound by covalent chemical bond, The International Union of Pure and Applied Chemistry (IUPAC) have recommended scientific name 'polyethene' , because IUPAC give name ethylene as ethene.
In industrial name line mold engineer of plastic engineer polyethylene is common called by PE. Is same with PS for polystyrene.
Ethene or Ethylene Molecule
as we know ethene (IUPAC) or ethylene consist of C molecule and H molecule with CH2=CH2 or C2H4, ethene as very simple structure. picture at left side shown the monomer of ethylene.
the illustration of polyethylene you can see at picture below, one monomer of ethylene connected with other with a hundred monomer and become polyethylene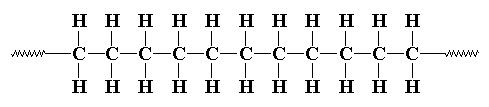
polyethylene chain picture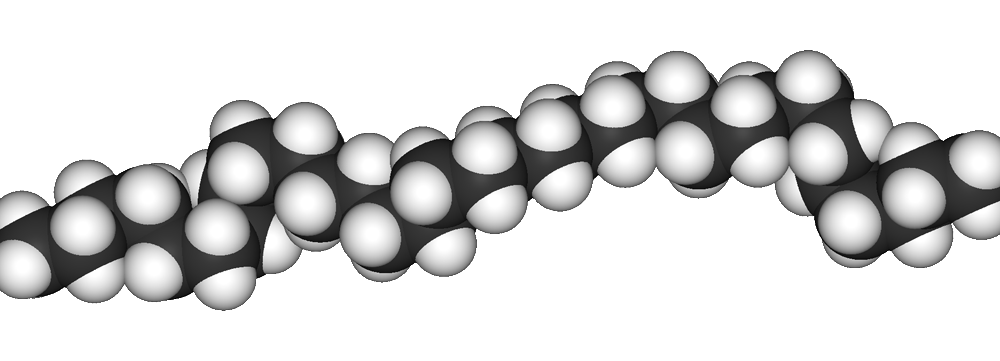
Polyethylene Industrially Practice
The first time polyethylene synthetical industrially practices was discovered by Eric Fawcett and Reginald Gibson at ICI Chemicals in 1933.
in 1935, another ICI chemist, Michael Perrin, start to develop a reproducible industrial synthesis for low density polyethylene (LDPE)
Using Catalyst
in 1951 by Robert Banks and John Hogan at Phillips Petroleum discover chromium trioxide based catalyst
In 1953, the German chemist Karl Ziegler developed a catalytic system based on titanium halides and organoaluminium compounds that worked at even milder conditions than the Phillips catalyst(http://www.otm-it.com/)
Producing polyethylene
how to produce polyethylene polymer ? Polyethylene both in industrially and laboratory produce by polymerization process, this process connected monomer or a mixture monomer become together by reaction that results a chain like form called polymer.
They are three methods that commonly to produce polyethylene in polymerization process
1. Radical polymerization
2. Anionic addition polymerization
3. Ion coordination polymerization (cationic addition polymerization )
Classification
The system of classification of polyethylene based on weight and density are
1. Ultra high molecular weight polyethylene (UHMWPE)
2. High molecular weight polyethylene (HMWPE)
3. High density polyethylene (HDPE)
4. High density cross-linked polyethylene (HDXLPE)
5. Medium density polyethylene (MDPE)
6. Low density polyethylene (LDPE)
7. Linear low density polyethylene (LLDPE)
8. Very low density polyethylene (VLDPE)
9. Ultra low molecular weight polyethylene (ULMWPE – PE-WAX)
10.Cross-linked polyethylene (PEX)
Polyethylene Characteristics
This material is known for its purity, elevated hardness, surface shine, resistance to abrasion, good rigidness and, in general, its excellent resistance to chemical agents. Its temperature of use can reach up to 110°C.
JIS testing Method
using standard test K6911 and K7112, this test equivalent with ASTM testing method D792, the results is :
Molding Shrinkage Rate (%)
LDPE shrinkage rate = 1.5 – 5.0
MDPE shrinkage rate = 1.5 – 5.0
HDPE shrinkage rate = 2.0 – 6.0
Compression Molding Pressure (kgf/cm2)
LDPE = 7.03 – 56.2
MDPE = 7.03 – 56.2
HDPE = 0.35 – 0.56
Injection Molding pressure (kgf/cm2)
LDPE = 562 – 2110
MDPE = 562 – 2110
HDPE = 703 – 1410
Plastic and Polymer Technology and Engineering Hot Topics
Abbreviations and Acronyms of Various Plastic Material
Posted by hasnan | | Abbreviations of PlasticsPlastics have various type, and each various type have they own name and they Abbreviations or Acronyms, here the list of Plastics material Abbreviations
or you should download the abbreviations here
ABS = Acrylonitrile-butadiene-styrene
ABA = Acrylonitrile-butadiene-acrylate
AES = Acrylonitrile-ethylene-styrene
ARP = Aromatic polyester
AS = Acrylonitrile-styrene
CA = Cellulose acetate
CMC = Carboxymethyl cellulose
ACM = Poly(Acrylic Acid Ester Rubber)
ACS = Acrylonitrile-Chlorinated Polyethylene-Styrene Terpolymer
AMMA = Poly(Acrylonitrile Methyl Methacrylate)
AN = Acrylonitrile
AO = Antioxidant
APET = Amorphous Polyethylene Terephthlate
ARP = Poly(Arylterephthalate) Copolyester
AS = Antistatic
ASA = Poly(Acrylic Styrene Acrylonitrile)
BDMA = Benzyl Dimethyl Amine (Epoxy Cure Accelerator)
BGE = Butyl Glycidyl Ether
BIIR = Bromobutyl Rubber
BMC = Bulk Molding Compound
BMI = Bismaleimide
BOPP = Biaxially Oriented Polypropylene (Film)
BR = Polybutadiene Rubber
CAB = Cellulose Acetate Butyrate
CAP = Cellulose Acetate Propionate
CF = Cresol Formaldehyde
CGE = Cresol Glycidyl Ether
CHDM = Cyclohexanedimethanol
CIIR = Chlorobutyl Rubber
CM = Chlorinated Polyethylene Rubber
CM = Compression Molded
CMC = Carboxymethyl Cellulose
CN = Cellulose Nitrate
CO = Epichlorohydrin Rubber (Homopolymer)
COF = Coefficient of Friction
CP = Cellulose Propionate
CPE = Chlorinated Polyethylene
CPVC = Chlorinated Polyvinyl Chloride
CR = Polychloroprene Rubber
CS = Casein
CSM = Chlorosulfonated Polyethylene Rubber
CTE = Coefficient of Thermal Expansion
CTFE = Chlorortrifluoroethylene
DAP = Diallyl Phthalate
DDS = Diaminodiphenyl Sulfone (Epoxy Cure Agent)
DGEBA = Diglycidyl Ether of Bisphenol A
EAA = Ethylene/Acrylic Acid Copolymer
EBAC = Poly(Ethylene Butyl Acrylate)
EC = Ethyl Cellulose
ECN = Epoxy Cresol Novolac
ECO = Epichlorohydrin Rubber (Ethylene Oxide Copolymer)
ECTFE = Poly(Ethylene Chlorotrifluoroethylene)
EEA = Poly(Ethylene-Ethyl Acrylate)
EEW = Epoxy Equivalent Weight (Also called WPE)
EMAAA = Ethylene Acid Terpolymer
EMAC = Poly(Ethylene Methyl Acrylate)
EMCM = Ethylene Methyl Acrylate Cyclohexene Methyl Acrylate
EP = Epoxy; Epoxide
EPDM = Ethylene Propylene Terpolymer Rubber
EPM = Ethylene Propylene Copolymer
EPN = Epoxy Phenol Novolac
EPS = Expanded Polystyrene
ETFE = (Ethylene Tetrafluoroethylene)
EVA = Ethylene Vinyl Acetate Copolymer
EVAC = Ethylene-Vinyl Acetate Copolymer
EVAL = Ethylene-Vinyl Alcohol
EVOH = Ethylene Vinyl Alcohol
FEP = Fluorinated Ethylene Propylene
FF = Furan Formaldehyde
FMQ = Fluorosilicone Rubber
FPM = Fluorocarbon Rubber
FPVC = Flexible Polyvinyl Chloride
FR = Flame Retardant
FVMQ = Fluorosilicone Rubber
FZ = Fluorinated Polyphosphazene Rubber
GFR = Glass Fiber Reinforced
HDPE = High Density Polyethylene
HDT = Heat Deflection Temperature or Heat Distortion Temperature
HFP = Hexafluoropropylene
HIPS = High Impact Polystyrene
HNBR = Hydrogenated Nitrile Rubber
IIR = Butyl Rubber
LCP = Liquid Crystal Polymer
LDPE = Low Density Polyethylene
LLDPE = Linear Low Density Polyethylene
LMDPE = Linear Medium Density Polyethylene
MDPE = Medium Density Polyethylene
MEKP = Methyl Ethyl Ketone Peroxide
MF = Melamine-Formaldehyde
NBR = Nitrile Rubber
NHFR = Non-Halogen Flame Retardant
NHT = High Temperature Nylon
OPP = Oriented Polypropylene (Film)
OPS = Oriented Polystyrene (Film)
OSHA = Occupation Safety and Health Administration (US Government)
PA = Polyamide (Nylon)
PA = Polyacrylate
PAEK = Polyarylether
PAEK = Polyaryletherketone
PAI = Polyamide-Imide
PAMS = Alpha Methylstyrene
PAN = Polyacrylonitrile
PARA = Polyarylamide
PAS = Polyarylsulfone
PASA = Polyamide, Semi-Aromatic
PASU = Polyarylsulfone
PB = Polybutadiene
PB = Polybutene-1
PBGA = Plastic Ball Grid Array
PBI = Polybenzimidazole
PBT = Polybutylene Terephthalate
PC = Polycarbonate
PCT = Polycyclohexylenedimethylene Terephthalate
PCTFE = Polychlorortrifluoroethylene
PCTG = Glycol-Modified PCT
PCU = Polycarbonate Urethane
PDAP = Poly(Diallyl Phthalate)
PDMS = polydimethylsiloxane (Silicone)
PE = Polyethylene
PEBA = Polyether Block Amide
PEEK = Polyetheretherketone
PEG = Polyethylene Glycol
PEI = Polyetherimide
PEK = Polyetherketone
PEKEKK = Polyetherketoneetherketoneketone
PEKK = Polyetherketoneketone
PEN = Polyethylene Naphthalate
PEO = Poly(Ethylene Oxide)
PEOX = Poly(Ethylene Oxide)
PES = Polyethersulfone
PESU = Polyethersulfone
PET = Polyethylene Terephthalate
PETG = PET Modified with CHDM
PEX = Cross-linked Polyethylene
PF = Phenol Formaldehyde (Phenolic)
PFA = Perfluoroalkoxy
PFPE = Polyperfluoropolyether
PI = Polyimide
PIB = Polyisobutylene
PIR = Polyisocyanurate Foam
PISU = Polyimidesulfone
PMMA = Polymethylmethacrylate
PMP = Polymethylpentene
PNR = Polynorborane Rubber
PO = Polyolefin
POM = Polyoxymethylene (Acetal)
PP = Polypropylene
PPA = Polyphthalamide
PPE = Polyphenylene Ether
PPF = Phenol-Furfural
PPG = Polypropylene Glycol
PPO = Polyphenylene Oxide
PPOX = Polypropylene Oxide
PPS = Polyphenylene Sulfide
PPSU = Polyphenylsulfone
PRF = Plastics Recovery Facility
PS = Polystyrene
PSS = Polysupersulfone
PSU = Polysulfone
PTFE = Polytetrafluoroethylene
PTMG = Polytetramethylene Glycol
PTT = Polytrimethylene Terephthalate
PU = Polyurethene
PUR = Polyurethene
PVAC = Poly(Vinyl Acetate)
PVAL = Poly(Vinyl Alcohol)
PVB =Poly(Vinyl Butyral)
PVC = Polyvinyl Chloride
PVCA = Poly(Vinyl Chloride-Acetate)
PVDC = Polyvinylidene Chloride
PVDF = Polyvinylidene Fluoride
PVFM = Poly(Vinyl Formal)
PVK = Polvinylcarbazole
PVOH = Polyvinyl Alcohol
PVP = Polyvinylpyrrolidone
PZ = Polyphosphazene Rubber
RH = Relative Humidity
RIM = Reaction Injection Molding
RPVC = Rigid Polyvinyl Chloride
RRIM = Reinforced Reaction Injection Molding
RTI = Relative Thermal Index (UL test)
RTPU = Rigid Thermoplastic Polyurethane
RTV = Room Temperature Vulcanizing (Silicone)
SAN = Poly(Styrene Acrylonitrile)
SB = Styrene-Butadiene
SBC = Styrene-Butadiene Copolymer
SBS = Poly(Styrene Butadiene Styrene)
SEBS = Poly(Styrene-Ethylene-Butadiene-Styrene) Elastomer
SIS = Poly(Styrene-Isoprene-Styrene) Elastomer
SMA = Poly(Styrene Maleic Anhydride)
SMMA = Styrene Methyl Methacrylate Copolymer
SMS = Styrene-a-Methylstyrene
SPS = Syndiotactic Polystyrene
SPU = Segmented Polyurethane
TAIC = Triallyl Isocyanurate
TEEE = Thermoplastic Elastomer Ether Ester Block Copolymer
TEEE = Ether Ester Block Copolymer (Thermoplastic Elastomer)
TEO = Olefinic Thermoplastic Elastomer
TES = Thermoplastic Styrenic Elastomer
TFE = Polytetrafluoroethylene
TP = Thermoplastic
TPE = Thermoplastic Elastomer
TPI = Thermoplastic Polyimide
TPO = Thermoplastic Polyolefin (often applied to elastomers)
TPU = Thermoplastic Polyurethene (often applied to elastomers)
TPUR = Thermoplastic Polyurethene (often applied to elastomers)
TPV = Thermoplastic Vulcanizate
TS = Thermoset
UF = Urea Formaldehyde
UHMW = Ultra High Molecular Weight (often applied to polyethylene)
ULDPE = Ultra Low Density Polyethylene
UP = Unsaturated Polyester (Thermoset)
VCE = Poly(Vinyl Chloride-Ethylene)
VCEMA = Poly(Vinyl Chloride-Ethylene-Methyl Acrylate)
VCMA = Poly(Vinyl Chloride-Methyl Acrylate)
VCVAC = Poly(Vinyl Chloride-Vinyl Acrylate)
VCVDC = Poly(Vinyl Chloride-Vinylidene Chloride)
VHMW = Very High Molecular Weight (often applied to polyethylene)
WPE = Weight per Epoxide (also called EEW)
XLPE = Cross-linked Polyethylene
A wide range of composite structures are prepared from polymer resins combined with fibers.for a more extensive discussion of polymer composites. Laminated polymer structures consist of layers of fibrous material impregnated with and bonded together usually by a thermosetting resin to produce sheets, bars, rods, tubes, etc. The laminate may be decorative or industrial,the latter being of load-bearing mechanical or electrical grade.
Phenolic plastics
Phenolic plastics can be reinforced with paper, cotton fabric, asbestos paper fabric or felt, synthetic fabric, or wood flour. They are used for general-purpose mechanical and electrical parts. They have good mechanical and electrical properties
Epoxies
These are used for high-performance mechanical and electrical duties. Fillers used are paper, cotton fabric, and glass fiber.
Melamine
Fillers used for melamine are paper, cotton fabric, asbestos paper fabric, and glass fabric. Melamines have a hard, non scratch surface, superior electrical properties, and can be rendered in self-colors. They are used for insulators, especially in wet and dirty conditions, and for decorative and industrial laminates.
Polyimide
Polyimide is most often used with glass fabric as filler. Polyimides have superior thermal and electrical properties with a service temperature similar to that for silicones but with two to three times the strength and flexibility.
Polyester
This is normally used with glass fabric (the cheapest) filler. The mechanical and electrical properties are inferior to those of epoxy. It can be rendered in self-colors.
Silicone
Silicone is used with asbestos paper and fabric and glass fabric fillers for high-temperature applications (250ーC; intermittent use 300ーC). It has excellent electrical but inferior mechanical properties.
Tufnol
tufnol,is the trade name for a large range of sheet, rod, and tube materials using phenolic resin with paper and asbestos fabric and epoxy resin with glass or fabric.
These combine very high strength, good temperature and abrasion resistance, exceptional dimensional stability, and low coefficient of thermal expansion. They compete with nylon (but with many better properties) and with metal die castings (but are lighter). Chemical resistance is good except for strong acids. Typical applications are water-pump parts, pipe fittings, washing machines, car instrument hous- ings, bearings, and gears.
Acrylics (Methylmethacrylate, PMMA)
These are noted for their optical clarity and are available as sheet, rod, tubings, etc., as Perspex (U.K.) and Plexiglas (
Acrylonitrile-Butadiene-Styrene (ABS)
This combination of three monomers gives a family of materials which are strong, stiff, and abrasion resistant with notable impact-resistance properties and ease of processing. The many applications include pipes, refrigerator liners, car-instrument surrounds, radiator grills, telephones, boat shells, and radio and television parts. Available in medium, high, and very high impact grades.
Cellulosics
“Cellulose nitrate” is inflammable and has poor performance in heat and sunlight. Its uses are therefore limited. Cellulose acetate has good strength, stiffness, and hardness and can be made self-extinguishing. Glass-filled grades are made. Cellulose acetate-butyrate (CAB) has superior impact strength, dimensional stability, and service temperature range and can be weather stabilized. Cellulose proprionate (CP) is similar to CAB, but has better dimensional stability and can have higher strength and stiffness. Ethyl cellulose has better low-temperature strength and lower density than the others. Processing of cellulose plastics is by injection molding and vacuum forming. Applications include all types of moldings, electrical insulation, and toys.
Ethylene-Vinyl Acetate (EVA)
This material gives tough flexible moldings and extrusions suitable for a wide temperature range. Thematerial may be stiffened by the use of fillers and is also specially formulated for adhesives. Applications include all types of moldings, disposable liners, shower curtains, gloves, inflatables, gaskets, and medical tubing. The material is competitive with polyvinyl chloride (PVC), polyethene, and synthetic rubbers,
and is also used for adhesives and wax blends.
Fluorocarbons
This class of polymers, characterized by fluorine substitution, has outstanding chemical, thermal, and electrical properties and is characterized by the following four main classes of structures. Polytetrafluoroethylene (PTFE), known commercially as Teflon or Fluon, is the best-known material and resists all known chemicals, weather, and heat, has an extremely low coefficient of friction, and is “non-stick.” These materials are inert with good electrical properties. They are nontoxic, nonflammable, and have a working temperature range of –270 to 260°C. They may be glass filled for increased strength
and rigidity. They do not melt and they must be formed by sintering of powders. Applications include chemical, mechanical, and electrical components, bearings (plain or filled with glass and/or bronze), tubing, and vessels for “aggressive” chemicals.
Fluoroethylenepropylene (FEP), unlike PTFE, can be processed on conventional molding machines and extruded, but thermal and chemical resistance properties are not quite as good. Ethylenetetrafluoroethylene (ETFE) possess properties similar to but not as good as those of PTFE.However, the material exhibits a thermoplastic character similar to that of polyethylene which gives it a very desirable molding behavior.
Perfluoroalkoxy (PFA) is the fourth group of fluorinated polymers. These materials have the same excellent properties as PTFE, but the compound is melt processible and, therefore, suitable for linings for pumps, valves, pipes, and pipe fittings.
Ionomers
These thermoplastics are based on ethylene and have high melt strength, which makes them suitable for deep forming, blowing, and other similar forming processes. They are used for packaging, bottles,moldings for small components, tool handles, and trim. They have a high acceptance of fillers.
Polymethylpentene
Polymethylpentene (TPX) is a high-clarity resin with excellent chemical and electrical properties and the lowest density of all thermoplastics. It has the best resistance of all transparent plastics to distortion at high temperature — it compares well with acrylic for optical use, but has only 70% of its density. It is used for light covers, medical and chemical ware, high-frequency electrical insulation, cables, micro-wave oven parts, and radar components. It can withstand soft soldering temperatures.
Polyethylene Terephthalate
Polyethylene terephthalate (PETP) and modified versions thereof have high strength, rigidity, chemical and abrasion resistance, impact resistance in oriented form, and a low coefficient of friction. It is attackedby acetic acid and concentrated nitric and sulfuric acids. It is used for bearings, tire reinforcement,bottles, automotive parts, gears, and cams.
Polyamides (Nylons)
The polyamides are a family of thermoplastics, e.g., Nylon 6, Nylon 66, and Nylon 610, which areamong the toughest engineering plastics with high vibration-damping capacity, abrasion resistance,inherent lubricity, and high load capacity for high-speed bearings. They have a low coefficient of frictionand good flexibility. Pigment-stabilized types are not affected by ultraviolet radiation and chemicalresistance is good. Unfilled nylon is prone to swelling due to moisture absorption. Nylon bearings maybe filled with powdered molybdenum disulfide or graphite. Applications include bearings, electrical
insulators, gears, wheels, screw fasteners, cams, latches, fuel lines, and rotary seals.
Polyethylene
Low-density polyethylene (orinally called polythene) is used for films, coatings, pipes, domestic mold-ings, cable sheathing, and electrical insulation. High-density polyethylene is used for larger moldingsand is available in the form of sheet, tube, etc. Polyethylene is limited as an engineering material because of its low strength and hardness. It is attacked by many oxidizing chemical agents and some hydrocarbonsolvents.
Polyketone, Aliphatic
Aliphatic polyketones are relatively strong, tough, ductile polymeric resins derived from equal propor-tions of ethylene and carbon monoxide with an additional few percent of higher olefin for property and processibility adjustment. Their physical, thermal, and mechanical properties are similar to polyamides and polyacetals. Mechanical properties are characterized by preservation of high levels of stiffness,
toughness, and strength over a broad temperature range. Resistance to hydrolysis, swelling, and perme-ation provides broad chemical resistance. Relatively new in commercial supply, they find application ingears, machine components, and similar engineering applications. Tribological performance is very good,and in particular they have a low coefficient of friction and a low wear factor against steel. The electrical
properties of the neat polyketone are typical of those of polar, semicrystalline thermoplastics.
Polyethersulfone
Polyethersulfone is a high-temperature engineering plastic — useful up to 180°C in general and some grades have continuous operating ratings as high as 200°C. It is resistant to most chemicals and may be extruded or injection molded to close tolerances. The properties are similar to those of nylons.Applications are as a replacement for glass for medical needs and food handling, circuit boards, general electrical components, and car parts requiring good mechanical properties and dimensional stability.
Polystyrene
This polymer is not very useful as an engineering material because of brittleness in unmodified forms, but it is well known for its use in toys, electrical insulation, refrigerator linings, packaging, and numerous commercial articles. It is available in unmodified form as a clear transparent resin and also in clear and opaque colors. High-impact forms are achieved by compounding with butadiene or other rubbery resins and heat-resistant forms are achieved by the use of fillers. Polystyrene can be stabilized against ultraviolet radiation and also can be made in expanded form for thermal insulation and filler products. It is attacked by many chemicals, notably aromatic hydrocarbon solvents, and by ultraviolet light.
Polysulfone
Polysulfone has properties similar to nylon, but these properties are retained up to 180°C compared with 120°C for nylon, which greatly expands the range of applications. Its optical clarity is good and its moisture absorption lower than that of nylon. Applications are as a replacement for glass for medicalneeds and chemistry equipment, circuit boards, and many electrical components.
Polyvinyl Chloride
This is one of the most widely used of all plastics. With the resin mixed with stabilizers, lubricants, fillers, pigments, and plasticizers, a wide range of properties is possible from flexible to hard types, in transparent, opaque, and colored forms. It is tough, strong, with good resistance to chemicals, good low- temperature characteristics and flame-retardant properties. PVC does not retain good mechanical per-formance above 80°C. It is used for electrical conduit and trunking, junction boxes, rainwater pipes and gutters, decorative profile extrusions, tanks, guards, ducts, etc.
Polycarbonate
Polycarbonate is an extremely tough thermoplastic with outstanding strength, dimensional stability, and electrical properties, high heat distortion temperature and low-temperature resistance (down to –100°C).It is available in transparent optical, translucent, and opaque grades (many colors). Polycarbonates have only fair resistance to chemicals as evidenced by the stress cracking caused by many solvents. The weathering tendencies can be stabilized against ultraviolet radiation by the use of proper additives.
Polycarbonate compounds are used for injection moldings and extrusions for glazing panels, helmets, face shields, dashboards, window cranks, and gears. Polycarbonate is an important engineering plastic.
Polypropylene
Polypropylene is a low-density, hard, stiff, creep-resistant plastic with good resistance to chemicals, good wear resistance, low water absorption, and is relatively low cost. Polypropylene can be spun into filaments, converted into weaves, injection molded, and is commonly produced in a large variety of forms. Glass-filled polypropylene is widely used for its enhanced mechanical properties. It is used for food and chemical containers, domestic appliances, furniture, car parts, twine, toys, tubing, cable sheath, and bristles.
Polyphenylene Sulfide
Polyphenylene sulfide is a high-temperature plastic useful up to 260°C. Ambient temperature properties are similar or superior to those of nylon. It has good chemical resistance and is suitable for structural components subject to heat. Glass filler improves strength and enables very high heat resistance to 300°C. Uses are similar to those of nylon, but for higher temperatures.
Polyphenylene Oxide
This is a rigid engineering plastic similar to polysulfone in uses. It can be injection molded and hasmechanical properties similar to those for nylon. It is used for automotive parts, domestic appliances,and parts requiring good dimensional stability. Frequently, the commercially available product is blended (or “alloyed”) with polystyrene which acts as a cost-effective extender.
Introduction
Plastics are polymers. What is a polymer? The simplest definition of a polymer is something made of many units. Think of a polymer as a chain. Each link of the chain is the "mer" or basic unit that is made of carbon, hydrogen, oxygen, and/or silicon. To make the chain, many links or "mers" are hooked or polymerized together. Polymerization can be demonstrated by linking strips of construction paper together to make paper garlands or hooking together hundreds of paper clips to form chains.
Polymers constitute a wide range of materials which are derived at least in part from organic, usually petroleum-based, raw materials; they consist of repeating molecular units and have special properties obtained by engineering the form of the molecular structures. The term polymer is derived from Greek roots and means “having many parts,” a term which aptly describes the infinite number of compounds which can be synthesized from a relatively limited number of monomer units. The term plastic is often used in describing polymers, although this term is not in current usage since it is a general descriptive
which refers to the forming rheology of many polymers but is too general to accurately describe this group of materials.
Polymers are used as engineering materials in the neat form, i.e., as the pure material, or in combination with a large diversity of additives, both organic and inorganic. These additives may be, among others, plasticizers which reduce the rigidity or brittleness of the material, fillers which increase strength andload deflection behavior under load, or stabilizers which protect the polymer against ultraviolet radiation.
The following discussion will separate polymers into two groups, thermoplastic and thermosetting, based on the distinctly different thermal processing behavior of these two broad classes of polymers.
Thermoplastic polymers soften when heated and can be reshaped, the new shape being retained oncooling. The process can be repeated many times by alternate heating and cooling with minimal degradation of the polymer structure. Thermosetting polymers (or thermosets) cannot be softened and reshaped by heating. They are plastic and moldable at some state of processing, but finally set to a rigid solid and cannot be resoftened. Thermosets are generally stronger and stiffer than thermoplastic.
Thermosetting Plastic
This type have Amorphous structure, During the irradiation of diamond by carbon atoms, amorphization of the crystal structure may occur and two specific amorphous forms of carbon may appear, he diamond like amorphous carbon which will be denoted by ta-C and the graphite like amorphous carbon named a-C. These two structures can be distinguish clearly by their macroscopic and microscopic properties
Types of Plastics
- Phenol (PF)
- Urea (UF)
- Melamine (MF)
- Polyester (UP)
- Epoxy (EPK)
Main Properties
- High distortion temperature, not softened any more by heating once set,
- Hardness is high generally
- Molded by transfer molding and compression molding in many case
Main Uses
This type of plastic can divide into 2 structure of plastic they are :
- Crystalline structure
- Amorphous structure
Crystalline structure
This type of plastic that have crystalline structure include :
- Polyethylene (PE)
- Polypropylene (PP)
- Polyamide (PA)
- Polyacetal (POM)
- Polyethylene teraphthalate (PET)
- Polypropylene sulfide (PPS)
Main Properties
- Has chemical resistance
- Adhesion and paintability are low
- Molding shrinkage is large so it is difficult to secure precise dimension
- Warp and sink are made easily and mold is not copied well
- Transparency is low
Main uses
- Tableware and containers
- Electronic parts that not need very precise dimension
- Sliding parts, machine parts
- Mechanism parts, connector coil bobbin
- Same frame and chassis
Amorphous structure
This type of plastic that have amorphous structure include
- Polystyrene (PS)
- ABS
- Acrylic resin (PMMA)
- Polysulfone (PSF)
- Polyvinyl chloride (PVC)
- Polysulfone (PSF)
- Polyurethane (PU)
Main Properties
- Chemical resistance is not high
- Adhesion and paint ability are high
- In Molding shrinkage is little so precision can be obtained more easily than crystalline plastics.
- Have high transparency
Main uses
- Miscellaneous goods and containers
- Electronic parts that need precise dimension
- Housing glasses
- Lenses and glasses
- Camera parts
- Watch band, soles
- Medical device parts
- Pipe and chassis that need transparency
The most plastic are made from petroleum, although natural gas and coal are used partly,
Naphtha, naphtha is a group of various liquid hydrocarbon intermediate oil refining products
used primarily as feedstocks for producing a high octane gasoline component via the catalytic
reforming process. Naphtha is also used in the petrochemical industry for producing olefins in
steam crackers and in the chemical industry for solvent applications.Then naphtha is decomposed thermally into monomers, which are converted into polymer by
polymerization, polymerization means a chain reaction the monomer to bond together to from a long chain of the molecules become high molecular compounds Plastic Classification Generally plastic can classified as shown below
Thermoplastic
A thermoplastic is a material that is plastic or deformable, melts to a liquid when heated and
freezes to a brittle, glassy state when cooled sufficiently. Most thermoplastics are high
molecular weight polymers whose chains associate through weak van der Waals forces
(polyethylene); stronger dipole-dipole interactions and hydrogen bonding (nylon); or even
stacking of aromatic rings (polystyrene). Thermoplastic polymers differ from thermosetting
polymers (Bakelite; vulcanized rubber) which once formed and cured, can never be remelted and remolded.
Many thermoplastic materials are addition polymersList kind of thermoplastics
• Acrylonitrile butadiene styrene (ABS)
• Acrylic
• Celluloid
• Cellulose acetate
• Ethylene-Vinyl Acetate (EVA)
• Ethylene vinyl alcohol (EVAL)
• Fluoroplastics (PTFEs, including FEP, PFA, CTFE, ECTFE, ETFE)
• Ionomers
• Kydex, a trademarked acrylic/PVC alloy
• Liquid Crystal Polymer (LCP)
• Polyacetal (POM or Acetal)
• Polyacrylates (Acrylic)
• Polyacrylonitrile (PAN or Acrylonitrile)
• Polyamide (PA or Nylon)
• Polyamide-imide (PAI)
• Polyaryletherketone (PAEK or Ketone)
• Polybutadiene (PBD)
• Polybutylene (PB)
• Polybutylene terephthalate (PBT)
• Polyethylene terephthalate (PET)
• Polycyclohexylene dimethylene terephthalate (PCT)
• Polycarbonate (PC)
• Polyhydroxyalkanoates (PHAs)
• Polyketone (PK)
• Polyester
• Polyethylene (PE)
• Polyetheretherketone (PEEK)
• Polyetherimide (PEI)
• Polyethersulfone (PES)- see Polysulfone
• Polyethylenechlorinates (PEC)
• Polyimide (PI)
• Polylactic acid (PLA)
• Polymethylpentene (PMP)
• Polyphenylene oxide (PPO)
• Polyphenylene sulfide (PPS)
• Polyphthalamide (PPA)
• Polypropylene (PP)
• Polystyrene (PS)
• Polysulfone (PSU)
• Polyvinyl chloride (PVC)
• Polyvinylidene chloride (PVDC)
• Spectralon
Thermosetting
plastics are polymer materials that cure, through the addition of energy, to a stronger form. The energy may be in the form of heat (generally above 200 degrees Celsius), through a chemical reaction (two-part epoxy, for example), or irradiation.Thermoset materials are usually liquid, powder, or malleable prior to curing, and designed to be
molded into their final form, or used as adhesives.The curing process transforms the resin into a plastic or rubber by a cross-linking process.
Energy and/or catalysts are added that cause the molecular chains to react at chemically active
sites (unsaturated or epoxy sites, for example), linking into a rigid, 3-D structure. The cross
-linking process forms a molecule with a larger molecular weight, resulting in a material with a
higher melting point. During the reaction, when the molecular weight has increased to a point so
that the melting point is higher than the surrounding ambient temperature, the material forms
into a solid material. Subsequent uncontrolled reheating of the material results in reaching the
decomposition temperature before the melting point is obtained. A thermo set material cannot be melted and re-shaped after it is cured.Thermo set materials are generally stronger than thermoplastic materials due to this 3-D network of bonds, and are also better suited to high-temperature applications up to the decomposition temperature of the material. They do not lend themselves to recycling like thermoplastics, which can be melted and re-molded.
Crystalline plastic
Some molecules of linear molecule compound can gather regulary, this type of plastic called the
crystalline plastic, not all the molecules crystallized, and the ratio of crystalline depend on
cooling condition, pre heating and others, sample of this type plastic are :
• Polyacetal resin (POM)
• Polymide resin (PA)
• Polypropylene resin (PP)
Armorphous plastic
Unlike crystalline plastic, the molecules of these plastic cannot gather regulary,this plastic
which is not crystalline, generally transmits light and has low solvent resistance sample of
this type plastic are :
• All the thermosetting plastic
• acrylic resin (PMMA)
• polystyrene (PS)
Polymer
A polymer is a substance composed of molecules with large molecular mass consisting of repeating structural units, or monomers, connected by covalent chemical bonds. its compounds obtained by polmerization, they are the base of plastics, if they are mixed with fillers, stabilizers they mixtures became moldable plasticspolimer can divide into-A homopolymer molecule is derived from a single monomer species, such as polyethylene or polymethylmethacrylate.-A copolymer molecule is derived from two or more monomer species, such as ethylene-vinyl
acetate or DNA. Depending on the arrangement of the individual monomers, a copolymer molecule may be classified by terms such as alternating, random, or statistical.
Manomer
is a small molecule that may become chemically bonded to other monomers from which polymers can be made, its a raw material of polymer this process also known as polymerization. a molecule of any of a class of compounds from polimer, mostly organic, that can react with other molecules of the same or other compound to form very large molecules, or polymers. The essential feature of a monomer is polyfunctionality, the capacity to form chemical bonds to at least two other monomer molecules Examples of monomers are hydrocarbons such as the alkene and arene homologous series. Here hydrocarbon monomers such as phenylethene and ethene form polymers used as plastics like poly(phenylethene) (commonly known as polystyrene) and poly(ethene) (commonly known as polyethylene
or polythene). Other commercially important monomers include acrylic monomers such as acrylic acid, methyl methacrylate, and acrylamide
Copolymer
a polymer consisting of two or more different monomers, The structural units derived from the different monomers may be present in regular alternation or in random order, Commercially relevant copolymers include ABS plastic, SBR, styrene-isoprene-styrene (SIS) and ethylene-vinyl acetate.
Homopolymer
A polymer formed from a single monomer, an example is polyethylene, formed by polymerization of ethylene A homopolymer is constructed of identical monomers or macromolecules consisting of a single type of building unit, his is in contrast to a copolymer where the polymer contains at least two monomers. It is frequently referred to simply as a polymer
What plastic are ?
Plastic covers a range of synthetic or semisynthetic polymerization products. They are composed of organic condensation or addition polymers and may contain other substances to improve performance or economics. There are few natural polymers generally considered to be "plastics". Plastics can be formed into objects or films or fibers. Their name is derived from the fact that many are malleable, having the property of plasticity
Time Line History
There are 3 big historical plastic area, begin at 1839 when processing of natural rubber invented by Charles Goodyear
Basic Natural Rubber
- 1839 - Natural Rubber - method of processing invented by Charles Goodyear
- 1843 - Vulcanite invented by Thomas Hancock
- 1843 - Gutta-Percha invented by William Montgomerie
- 1856 - Shellac invented by Alfred Critchlow and Samuel Peck
- 1856 - Bois Durci invented by Francois Charles Lepag
- 1839 - Polystyrene or PS discovered by Eduard Simon
- 1862 - Parkesine In London, Alexander Parkes unveils the first-ever man-made plastic. Dubbed "Parkesine," it fails due to high costs
- 1863 - Cellulose Nitrate or Celluloid discovered by John Wesley Hyatt
- 1069 - XyloniteAfter the failure of Parkesine, Daniel Spill tries to manufacture a similar material named Xylonite. The company goes bankrupt in 1874
- 1872 - Polyvinyl Chloride or PVC first created by Eugen Baumann
- 1894 - Viscose Rayon discovered by Charles Frederick Cross and Edward John Bevan
Thermosetting Plastics and Thermoplastics
- 1908 - Cellophane discovered by Jacques E. Brandenberger
- 1909 - First true plastic Phenol are Formaldehyde tradenamed Bakelite. Bakelite The first completely synthetic man-made substance, Bakelite is invented in 1909 by independent New York chemist Leo H. Baekeland. The "material of a thousand uses" is used to make everything from car parts to jewellery, but jewellery sales are suspended in 1942 in order to concentrate supplies on the war effort. Bakelite pieces are now valuable collectibles. Andy Warhol was an avid collector and, when he died in 1987, his pieces sold for record prices at Sotheby's1926 - Vinyl or PVC discovered by Walter Semon invented a plasticized PVC.
- 1927 - Catalin, When Bakelite's 1910 patent expires in 1927, the Catalin corporation starts making the same substance under the name "Catalin" and adds fifteen new colours to the colour range. 70% of the "bakelite" remaining today is Catalin. Also suspends jewellery sales in 1942 (see above). Plastic is the perfect medium for the Art Deco period, when bold, colourful, geometric designs are popular.
- 1927 - Cellulose Acetate
- 1933 Polyvinylidene chloride or Saran also called PVDC accidentally discovered by Ralph Wiley, a Dow Chemical lab worker.
- 1935 - Low-density polyethylene or LDPE discovered by Reginald Gibson and Eric Fawcett
- 1936 - Acrylic or Polymethyl Methacrylate was discovered
- 1937 - Polyurethanes tradenamed Igamid for plastics materials and Perlon for fibers discovered by Otto Bayer and co-workers discovered and patented the chemistry of polyurethanes
- 1938 - Polystyrene made practical
- 1938 - Polytetrafluoroethylene or PTFE tradenamed Teflon discovered by Roy Plunkett
- 1939 - Nylon and Neoprene considered a replacement for silk and a synthetic rubber respectively Wallace Hume Carothers
- 1941 - Polyethylene Terephthalate or Pet - Whinfield and Dickson
- 1942 - Low Density Polyethylene
- 1942 - Unsaturated Polyester also called PET patented by John Rex Whinfield and James Tennant Dickson
- 1951 - High-density polyethylene or HDPE tradenamed Marlex invented by Paul Hogan and Robert Banks
- 1951 - Polypropylene or PP invented by Paul Hogan and Robert Banks
- 1953 - Daniel Fox, a chemist at General Electric, discovers a polycarbonate resin thermoplastic that looks like acrylic but is much more durable (almost bulletproof). A patent is filed in 1955 and it is given the brand name "Lexan." Familiar products made of Lexan include Apple's iBook and iPod and Naglene water bottles.
- 1953 - Saran Wrap introduced by Dow Chemicals.
- 1954 - Styrofoam the trademarked form of polystyrene foam insulation, invented by Ray McIntire for Dow Chemicals
- 1964 - Polyimide
- 1970 - Thermoplastic Polyester this includes trademarked Dacron, Mylar, Melinex, Teijin, and Tetoron
- 1978 - Linear Low Density Polyethylene
- 1985 - Liquid Crystal Polymers wasdiscovered
and until now many ofkinf plastic was dicovered, now almost product good always contain a plastics




